Allergists in Norwalk and Shelton, CT
Personalized Care for Kids & Adults — Beat Pollen, Food Allergies and More
At the Allergy Center of Connecticut, we do more than treat allergies—we walk alongside you through every season, flare-up, and unknown. Whether you’re dealing with persistent sneezing that just won’t quit, mysterious food reactions, or chronic sinus issues that keep you up at night, we’re here to help you breathe easier, live fuller, and worry less.
With convenient locations in Norwalk and Shelton, our board-certified allergists bring decades of combined experience to families across the state. We take pride in delivering thoughtful, individualized
Meet Our Team of Connecticut Allergists
Our team includes a board-certified allergist and immunologist, and a physician assistant who bring together extensive clinical experience and a deep understanding of allergy-related conditions.
Dr. Philip Hemmers, DO, FAAAAI, has been practicing medicine for over 20 years. He specializes in environmental and food allergies, and is known for expertise and holistic approach.
Our physician assistant, Martin Ronan, offers informed care with a focus on identifying allergies and providing relief.
Comprehensive Allergy Treatments in Connecticut
Seasonal Allergies

- We test what’s triggering you (like birch or oak) and create custom plans—shots, drops, or rinses—to ease symptoms fast.
Food Allergies

We do precise tests (skin or blood) and offer oral immunotherapy (OIT) to help you tolerate foods safely over time.
Hives
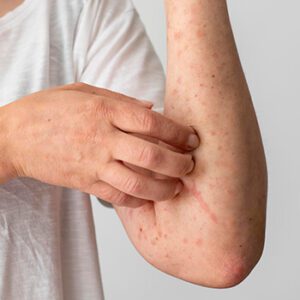
We dig deep with testing to find the cause and use meds or plans to stop them from coming back.
Asthma

We test your triggers, adjust treatments (like inhalers or biologics), and keep your lungs happy.
Other Allergy Issues

Things like pet dander, mold, or sinus troubles (e.g., congestion or polyps) that mess with your day.
From rinses to allergy shots, we’ve got options to fit your life.
Patient-Centered Care Approach
At the core of everything we do is a belief that you deserve to feel heard. We don’t rush appointments, rely on cookie-cutter plans, or make assumptions based on symptoms alone. Instead, we take the time to get to know you—your routines, your health history, your goals—so we can design care that actually fits your life.
Our allergy centers in Shelton and Norwalk, Connecticut prioritize clear communication and shared decision-making. We want you to understand your diagnosis, feel confident about your options, and feel fully supported in every step of your care.
Patient Resources
We know that managing allergies or immune issues isn’t always straightforward—and that it’s easy to feel overwhelmed by conflicting advice online. That’s why we’ve built out a robust library of patient resources to guide you with clarity and confidence.
Your visit:
MyChart access forms:
Why Choose Us?
Fast Testing for Pollen & Food Triggers

Custom Plans — including Shots and Drops
Smart Tools to Predict Flare-Ups
Care for All Ages, from Kids to Adults
Schedule an Appointment With Our Connecticut Allergists
Whether you’re ready to schedule a visit or just have a few questions, we’re here to make it easy. You’ll find our allergy centers in Norwalk and Shelton, both conveniently located and designed with your comfort in mind.
Norwalk Office:
761 Main Ave, Suite 105
Norwalk, CT 06851
Phone: 203-870-8731
Shelton Office:
4 Corporate Dr, Suite 295
Shelton, CT 06484
Phone: 203-374-6103
Both offices offer free parking, easy highway access, and accessible exam rooms. Not sure which location is right for you? Give us a call—we’ll help you find the best fit.
What Our Clients Say
Trustindex verifies that the original source of the review is Google. Very efficient and professional care of patients. Dr. Ronan explains the procedures and answers question very clearly, easy to understand. The staff is very helpful and will make you feel at ease. Always pleasure to come back.Trustindex verifies that the original source of the review is Google. I just met with Martin Ronan, PA for some antibiotic testing. I was very pleased with his service and he was personable, gentle and nice to work with. I will follow up with further testing but am happy to recommend him and this office for those seeking allergy testing or treatment.Trustindex verifies that the original source of the review is Google. My daughter needed allergy testing for possible food intolerances. The front desk was so nice & Dr. Hemmer was very patient. Highly recommend!Trustindex verifies that the original source of the review is Google. Dr. Martin Ronan change my life! When I first stepped into his office three years ago, I had gotten so used to my allergies, asthma and my skin being in such terrible condition, I had learned to ignore it. He saw how uncomfortable I was and how my quality of life was horrible and immediately took care of me. Putting me on a regimen that changed my life and continues to follow up to see how I am doing until today.Trustindex verifies that the original source of the review is Google. Martin Ronan you’re excellent! I wish you the best of success! I will recommend you to family and friends. Being a good person and compassionate truly make someone their best at what they do. Thanks again for your help with my allergy!😊Trustindex verifies that the original source of the review is Google. Excellent Doctor and staff !
